2020 Blogs
12 of 166 items displayed

Berkeley Protocol on Digital Open Source Investigations
Matthew Renshaw and Lauren Chaplin discuss the Berkeley Protocol on Digital Open Source Investigations, which provides guidelines for using open-source intelligence (OSINT) in criminal and human rights investigations, and consider how law firms should adapt to OSINT’s potential.

Corporate pledges to address racial inequality: a cause of action?
Oliver Holland and Walker Syachalinga discuss the effects of corporate pledges in the wake of the Black Lives Matter campaign. Man signing a document

Coronavirus on the frontline: how the pandemic has left Addison Lee Drivers vulnerable
Private hire drivers have been on the frontline helping to keep the UK going during Coronavirus. Here, solicitor Liana Wood discusses how the pandemic has left Addison Lee drivers exposed.
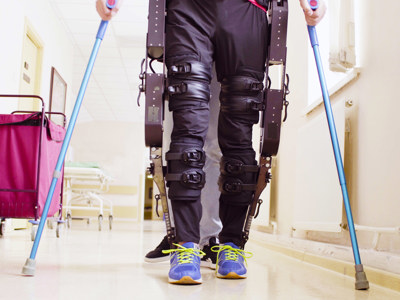
Personal injury rehab: Key steps for aiding seriously injured clients
David Preston explains the importance of personal injury rehabilitation and how it is managed during the personal injury claim process.

Looming emissions controversy for PHEVs
Shazia Yamin and Lucy Martin discuss revelations that plug-in hybrid electric vehicles (PHEVs) may not be emitting the low levels of polluting gases that consumers had been promised.

Prison service must strike a better balance on the use of handcuffs on prisoners in hospital
Benjamin Burrows and Ellie Sutherland discuss the use of handcuffs on prisoners accessing hospital treatment and argue that it is a balance that the prison service is repeatedly getting wrong.
Human rights issues highlighted by COVID-19
On Human Rights Day, Kate Egerton, Aisha Asghar and Abi Joseph look at how human rights are more important than ever in protecting our basic rights and freedoms in the context of the COVID-19 pandemic.

Review of gambling laws announced by government
Nichola Marshall and Paula Lee discuss the announcement of the government review of gambling laws.

“Healing crystals” with a trail of exploitation and violence
Rebecca Swan discusses the ethics of buying into the craze for healing crystals that could have been mined by children or be helping to finance war and terror.




